FDA finalizes rule expanding availability of abortion pills
Associated Press | 1/5/2023, 6 p.m.
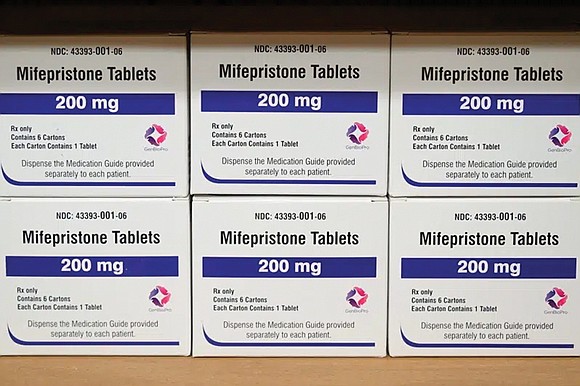
WASHINGTON - The Food and Drug Administration on Tuesday finalized a rule change that broadens availability of abortion pills to many more pharmacies, companies.
The Biden administration partially implemented the change last year, announcing it would no longer enforce a long-standing requirement that women pick up the medicine in person. Tuesday’s action formally updates the drug’s labeling to allow many more retail pharmacies to dispense the pills, so long as they complete a certification process.
The change could expand access at both brick-and-mortar stores and online pharmacies. Women can get a prescription via telehealth consultation with a health professional, and then receive the pills through the mail, where permitted by law.
Still, the rule change’s impact has been blunted by numerous state laws limiting abortion broadly and the pills specifically. Legal experts foresee years of court battles over access to the pills, as abortion-rights proponents bring test cases to challenge state restrictions.
For more than 20 years, the FDA labeling had limited dispensing to a subset of specialty offices and clinics, due to safety concerns. During the COVID-19 pandemic, the FDA temporarily suspended the in-person requirement. The agency later said a new scientific review by agency staff supported easing access, concurring with numerous medical societies that had long said the restriction wasn’t necessary.
Two drugmakers that make brand-name and generic versions of abortion pills requested the latest FDA label update. Agency rules require a company to file an application before modifying dispensing restrictions on drugs.
Danco Laboratories, which sells branded Mifeprex, said in a statement the change “is critically important to expanding access to medication abortion services and will provide healthcare providers” with another option for prescribing the drug.
The American College of Obstetricians and Gynecologists called the update an “important step” forward.
“Although the FDA’s announcement today will not solve access issues for every person seeking abortion care, it will allow more patients who need mifepristone for medication abortion additional options to secure this vital drug,” the group said.
More than half of U.S. abortions are now done with pills rather than surgery, according to the Guttmacher Institute, a research group that supports abortion rights.